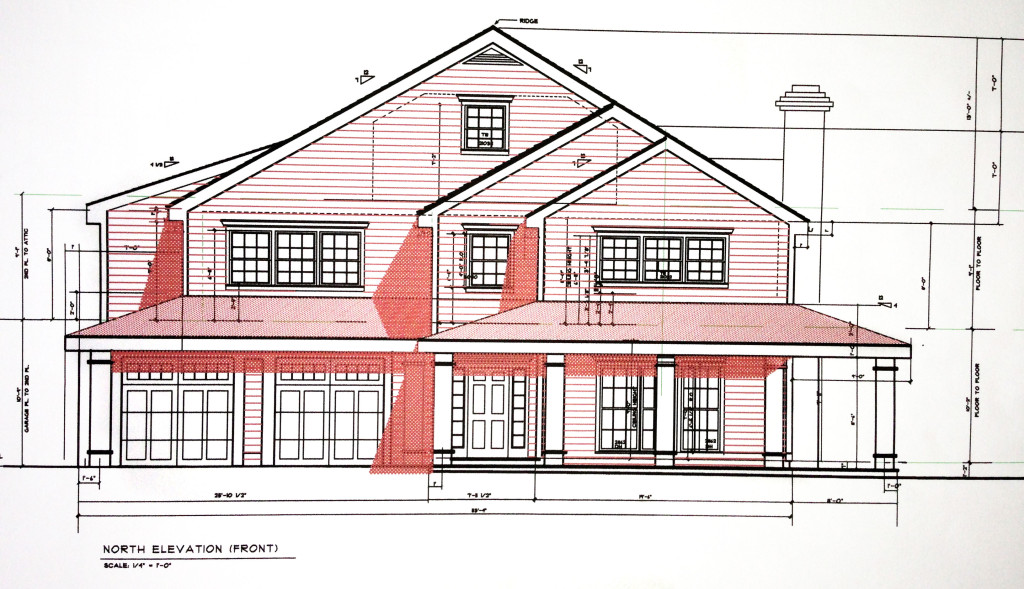
Private Residence – St. Mary’s by the Sea
Serving New York City, Westchester, and Connecticut
recent projects
New Construction
Lancaster Architecture, LLC provides comprehensive architectural services for both Residential and Commercial ground up new construction. Our body of work includes both modest and luxury homes, shopping centers, office... more»
sample clients





![]()
![]()
tell us
Call (203) 451-4638 we will listen to your wants and needs to bring your project to life—on budget.
about us
Lancaster Architecture LLC specializes in office interiors, medical and retail spaces. We work closely with both property owners and companies leasing new spaces to plan the most efficient and attractive work environments. The scope of our work includes conceptual design, design development and drawings suitable for permit, pricing and construction. Lancaster Architecture LLC provide services in NYC, Westchester, and throughout Connecticut. Read more
Accreditations



Licensed in CT, NY and FL; Nationally Accredited